HeX Group, your partner
Microbiological and particulate monitoring of environments classified as A, B, C or D is subject to high and specific levels of requirements. During a health authority inspection (FAMHP, FDA), many of the monitoring components can be verified. There are several factors to consider for microbiological monitoring of A, B, C and D environments
HeX Group accompanies you for each step
Phase 1: Documentary part
Successful monitoring requires a clear, precise, pragmatic and user-friendly documentation system. The QA department at HeX supports users* by providing different templates and accompanying them in their implementation:
– General monitoring template
– Template of the monitoring protocol per test, per class and per state (operating mode)
– Sampling plan template including risk analysis by zone (1 to 15 premises)
– Support in the creation and integration of the plans + 3 checks of the client’s documentary production (start / intermediate / end)
– Carrying out the various tests
* Subject to the order of the entire monitoring service.
Phase 2: Training
In the same way that the documentary system is the subject of particular attention during audits, the skills and qualifications of the “sampling” personnel must be justified.
In the continuity of the documentary system, HeX thus proposes its various training modules followed by an evaluation of practices:
Good practice in microbiological sampling
Good practice in particle counting
Evaluation of practices
Documentary traceability.

Phase 3: Equipment and consumables
HeX Group, through SAFYR, has selected the different equipment necessary to carry out particulate and microbiological monitoring in accordance with the various requirements.
Particle counters
Aerobiocollector
Culture media
Specificities for the culture media :
The culture media selected from “Merck” are the result of a comparative study to meet the requirements of the pharmacopoeia, but above all the constraints of the organisations linked to the services and/or the types of environment. Indeed, the proposed media depend on the targeted class (A, B, C, D), the type of installation (airlock, isolator, premises, PSM, etc.) and the type of strain to be identified. In addition, HeX Lab offers the possibility of benefiting from our sterility and fertility tests on each batch if the authorities so request. The other undeniable advantage in terms of management, organisation and cost constraints lies in the storage temperatures of the media. They can be kept at room temperature (15-25°C) = easy storage (no fridge, no probes, no metrology such as mapping & calibration) and no risk of condensation. These culture media have a long shelf life = ease of logistics and management of consumables.
Specificities for the SAFYR OPC particle counter: Consult us
Phase 4: Transport
HeX Group offers :
An internal solution for the collection of microbiological samples at the counter of the various customer services. Controlled and monitored transport conditions to guarantee our customers good conservation conditions during the journey. The guarantee of incubation time and risk control during transport are thus secured.

Phase 5: Laboratory skills

HeX LAB carries out the entire analytical part of the culture media resulting from customer samples
Double incubation of plates (20-25°C for bacterial flora and 30-35°C for fungal flora)
Reading by enumeration of the plates
Encoding of results
Photography in case of threshold exceedance
ISO 17025 and GMP documentation system to ensure compatibility with the requirements of the authorities (management of non-conformities, QTA, etc.)
HeX LAB’s activities also allow it to support the client in various ways:
– Identification of germs from the environment to genus and species using a genomic method (99% success rate)
The attached article on the comparative analysis of analysis methods between medical biology and a QC molecular biology lab helps to identify this risk.
– Beyond the microbiological monitoring of environments, it is essential to note that controls on finished products are also required by the health authorities (FAMHP). It is therefore important to optimise and pool the skills of the partner laboratory so that it can also respond to this type of testing:
Sterility tests on all types of products
Microbial cleanliness tests (=bioburden)
Endotoxin control However, these tests on finished products are much more restrictive in terms of requirements for the laboratory than the analyses of environmental agars. Among other things, they require:
– Premises classified at the same level of requirements as the production or preparation of the products (class A)
– All the organisation expected in the GMP (Good Manufacturing Practice)
– Validation of results by a QP (Qualified Person) pharmacist All these requirements are already met by HeX LAB and recognition as a QC GMP laboratory is expected by June 2021. This ultimate guarantee that all results provided by HeX LAB will be accepted by the inspecting health authorities.
– Validation of the effectiveness of cleaning and disinfection methods This point, which is eagerly awaited by the authorities, also requires specific expertise. HeX, by combining many of the elements presented above (risk analysis, microbiological ecology, biocidal efficacy tests, etc.), offers a pragmatic approach that is adapted to the resources and organisations of departments subject to hospital-type GMP.
Phase 6: Traceability and analysis of DATA
Sample coding
Follow-up sheet
Encoding of results
Trend analysis.
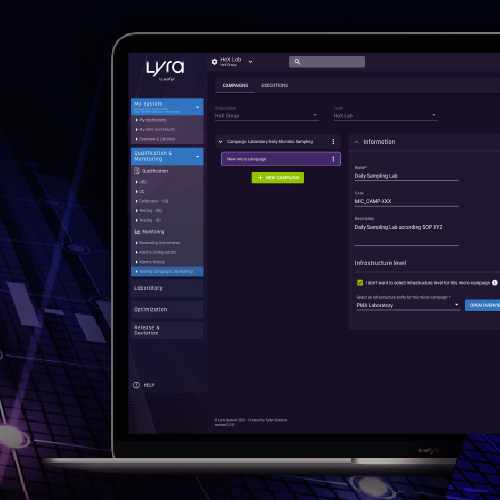
HeX in relation with SAFYR proposes a LYRA software solution allowing the implementation and the follow-up of a monitoring on a single support.
The Cleanrooms webinar of 20/05 precisely evokes this critical point often the “Achilles heel” of non-industrial GMP departments such as hospitals.
The LYRA® software offers :
A “Premium” MONITORING module allowing :
The creation of a microbiological sampling campaign from the risk analysis
Intelligent microbiological sampling sheets.
A LABORATORY module allowing :
To follow the course of the culture media according to the different stages (reception / incubation / reading / storage / disposal).
To encode results.
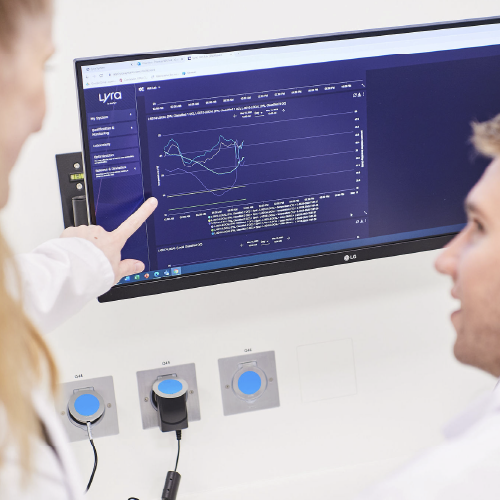
A My SYSTEM module allowing :
To visualize all the results (raw / graphical…)
To analyse trends
To be alerted in case of threshold overrun
Learn more about LYRA®